2D materials for photoelectrochemical water-splitting
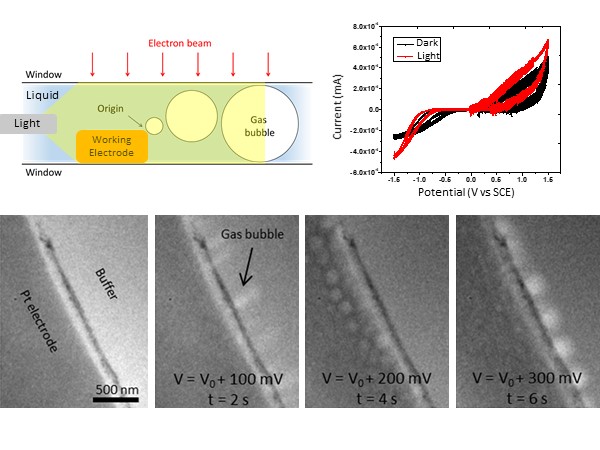
Photoelectrochemistry provides a promising, environmentally friendly route to hydrogen production; however, the atomic-scale mechanisms of the photocatalysts that facilitate the water-splitting reaction are currently poorly understood. Researchers at the University of Pennsylvania have studied the photocatalytic activity of several 2D nanomaterials such as gold nanoprisms and MoS2 flakes. The research focused on the correlation between I-V characteristics with water splitting and simultaneous structural changes at the catalytically active sites. The I-V characteristic curve with the illuminated catalyst shows additional activity in the oxidation regime when operating in in-situ illuminated conditions. In contrast, such activities are not observed in the absence of the catalytic particles. TEM images show the formation of gas bubbles induced at the working electrode with the illumination of the catalysts corresponding to water splitting.
Image Right – Top: Schematic shows the optical setup in liquid cell and redox IV characteristics of active catalytic material with and without light. Bottom: In-situ TEM images show corresponding hydrogen evolution and bubble formation at the electrode-electrolyte interface during the hydrogen evolution reaction (HER). From left to right, the evolution of bubble formation is captured as a function of time and applied potential.
HBS internal data obtained in collaboration with Pawan Kumar, Deep Jariwala and Eric Stach at the University of Pennsylvania.