In a collaborative effort between Toyota, LBNL, UCSC and Hummingbird Scientific, the researchers have combined operando electrochemical-synchrotron soft X-ray absorption (sXAS) and transmission electron microscopy (TEM) to study magnesium deposition and dissolution in borohydride-based electrolytes. The study shows for the first time that magnesium batteries function with SEI layer on the anode surface, contradicting the previous assumption that magnesium deposition and dissolution can only be achieved without surface films. These findings are published in the latest issue of Chemistry of Materials.
Copyright © 2017 ACS
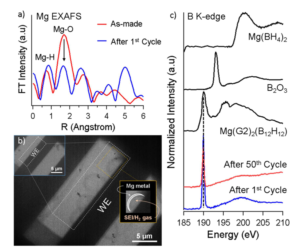
Using Hummingbird cross-platform liquid cell technology, the researchers first used Extended X-Ray Absorption Fine Structure (EXAFS) to investigate the solvent coordination of the as-prepared Mg(BH4)2: 3LiBH4/DME electrolyte and after one reduction/oxidation cycle. The decrease in the intensity of Mg-O bond after the first cycle suggested the loss in solvent coordination at the interphase. Upon studying the oxidation/reduction processes in liquid TEM, they observe the formation of passivating surface SEI layer and H2 gas beneath the magnesium metal deposit. Further study of B K-edge XAS indicated that [BH4]- anion promoted the reductive formation of H2 gas. These results present a new paradigm into using magnesium borohydride-based electrolytes for rechargeable magnesium batteries.
Learn more about the Liquid Cell products used in this experiment:
In-Situ TEM Liquid-Electrochemistry Holder
Reference: Timothy S. Arthur, Per-Anders Glans, Nikhilendra Singh, Oscar Tutusaus, Kaiqi Nie, Yi-Sheng Liu, Fuminori Mizuno, Jinghua Guo, Daan Hein Alsem, Norman J. Salmon, and Rana Mohtadi. “Interfacial insight from operando sXAS/TEM for magnesium metal deposition with borohydride electrolytes,” Chemistry of Materials (2017). DOI: 10.1021/acs.chemmater.7b01189
View All News

